Each line in the spectra represents the energy


Measurement-induced collective vibrational quantum coherence under

The Periodic Table & Quantum Theory - ppt download

Atomic Structure Quiz #2 H 10/12/21 Flashcards
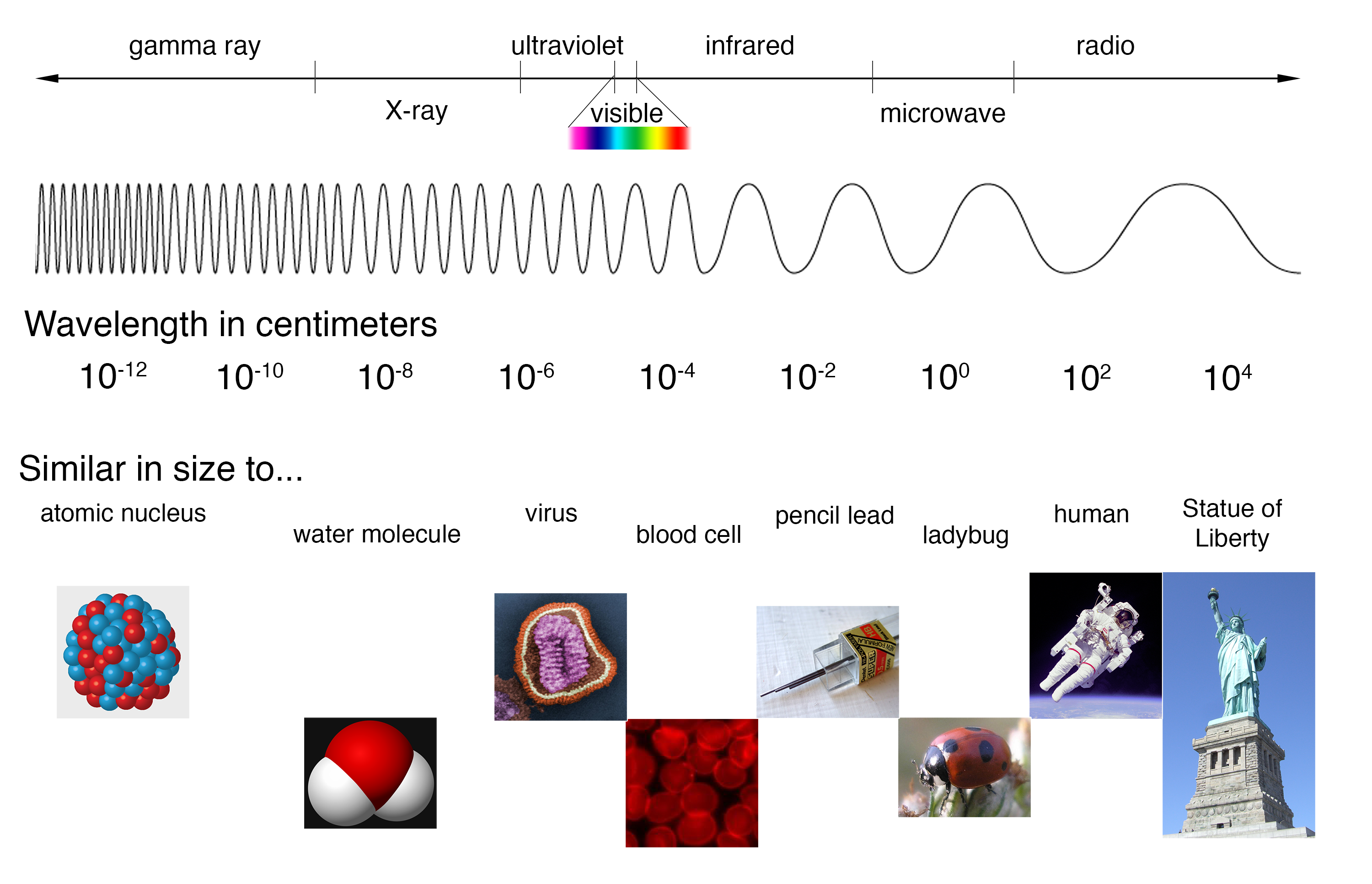
Spectra - Introduction

Kami Export - GENESIS PEREZ GIL - Emission Spectra WS.pdf - 1. 2. The characteristic bright-line spectrum of an element is produced when

The Periodic Table & Quantum Theory - ppt download

Each line in the spectra represents the energy

Why are atomic spectra of an element discontinuous?
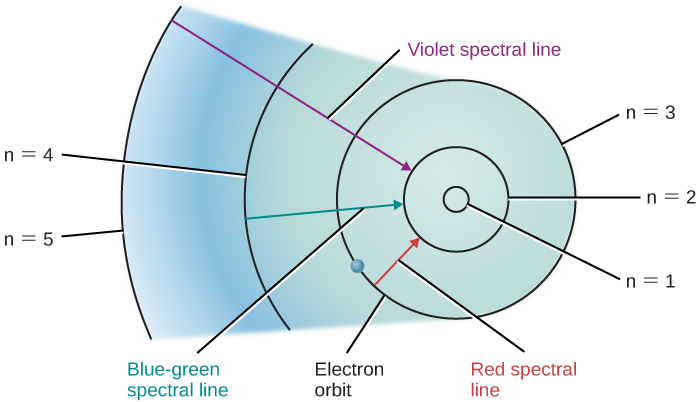
5.5 Formation of Spectral Lines – Astronomy
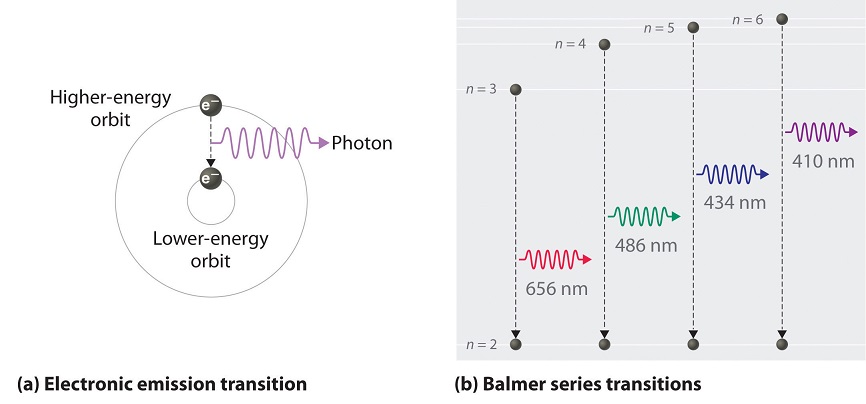
6.3: Line Spectra and the Bohr Model - Chemistry LibreTexts

SOLVED: Wavelength: 700 nm ACQ nm 450 nm 500 nm 550 nm 600 nm 650 nm Bright Line Spectrum (Gpts) Record the color and wavelength of the three strongest lines Normal B I

Energy Level: Definition, Equation (w/ Diagrams)

What do the lines in an emission spectrum represent? - Quora





